Researchers participating in the Blueprint Neurotherapeutics Network for Biologics receive grant funding (a UG3/UH3 award or U44 award if a small business) and no-cost access to biotherapeutic development consultants and contract research organizations (CROs).
Research Grants (UG3/UH3 or U44)
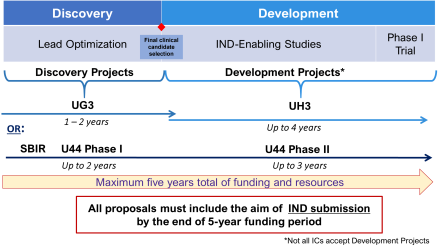
All projects that enter BPN-Biologics begin with a UG3 phase (or U44 Phase I) of up to two years, which should be used to complete all lead optimization activities (for Discovery-stage projects) or any additional preparatory activities needed to initiate IND-enabling studies (for Development-stage projects). This phase will also be used to engage the LDT in establishing a detailed research plan and go/no-go milestones for all subsequent work.
Projects can enter BPN-Biologics during the Discovery or Development stage and may seek support through phase I/first-in-human testing. Discovery involves biologic agent characterization and optimization in order to identify a suitable development candidate. During the Development stage, a candidate undergoes preclinical toxicology testing required for an IND and manufacturing, ultimately advancing to phase I/first-in-human testing. BPN-Biologics-supported Development activities include chemical manufacturing and controls (CMC), formulation development, toxicology studies, regulatory support, and phase I/first-in-human testing.
Consultants
Each Lead Development Team receives support from BPN-Biologics consultants. Consultants are assigned to a project with considerations for the project team's individual needs and consultants' expertise.
Current Consultants
Contract Research/Manufacturing Organizations
BPN-Biologics awardees receive no-cost access to the network's Contract Research Organizations (CROs). LDTs plan and coordinate studies conducted by BPN CROs.
- Image

- Image

- Image

Informational Resources
U44 mechanism comparison to other potential mechanisms for small business applicants
| Small Business Applicants | U44 mechanism comparison to other potential mechanisms |
| STTR and SBIR Omnibus program | BPN-Biologics |
| R43/R44 mechanism | U44 mechanism |
| Broad modalities of product development (including, but not limited to, small molecules, technologies, devices, biologics, etc.) | Limited to biologics development only (e.g., antibodies, peptides, proteins, oligonucleotide- and viral-based gene therapies, cell therapies, and other novel emerging therapies (e.g., microbial and microbiome therapies). |
| Indications are extremely broad | Indications are specified by participating institutes. |
| More competitive due to the number of applications | Not as competitive in terms of applications’ number |
| All stages of product development, no hard criteria for phase 1 entry. | Limited to the late stage of pre-clinical product development & optional clinical trials. |
| Budget is limited: SBA hard cap - Phase 1: $275,766 for (6 months-2 years) Phase 2: $1,883,436 (2-3 years) Waiver limits – Phase 1: $700,00 for (cap of $500,000/year) Phase 2: $3,000,000 (cap of $1,500,00) | Budget does not have a hard cap but should be within recommended frame: Phase 1: up to $500K/year (up to 2 years) Phase 2: up to 1.5M/year (no more than 3 years) |
| Granted funding | Granted funding + support (NIH experts, consultants and CROs contracted by NIH) |
| NIH scientific program staff do not collaborate on project activities after the award | After the award, NIH scientific program staff will assist, guide, coordinate, or participate in project activities |
| Milestones are only for Fast-Track mechanism (combined Phase I and Phase 2 award) | Milestones are set by Lead Development Team and must be met in order to move the project further. If milestones are not feasible to achieve, the project will be discontinued. |
| Can be used as a preparation stage prior the application to BPN-Biologics. |
| UG3/UH3 BPN-Biologics | U44 BPN-Biologics (SBIR Fast-track) |
| Eligibility: Higher Education Institutions, Non-profits, Local Governments, Federal Government Agencies, Small Businesses & Other For-Profit Organizations, Others. | Eligibility: Only U.S. small business concerns (SBCs) |
| Additional support is available through SEED program for small business aspects | |
Non-domestic (non-U.S.) Entities (Foreign Institutions) are eligible to apply. Non-domestic (non-U.S.) components of U.S. Organizations are eligible to apply. | Non-domestic (non-U.S.) Entities (Foreign Institutions) are not eligible to apply. Non-domestic (non-U.S.) components of U.S. Organizations are not eligible to apply. |
| No specific requirements for PD/PI primary employment | The Project Director/Principal Investigator (PD/PI) must have his/her primary employment with the small business concern at the time of award and for the duration of the project period |
| UG3/UH3 projects are 100% funded by BPN-Biologics program and might have limitations due to funds availability | The funding of the grant comes from SBIR program, a Federally mandated set-aside, which can only fund small businesses. |
| Data sharing is essential | Data sharing is not mandatory; the data are protected for 20 years. See SBIR data right FAQ . |
| A commercialization plan is developed and updated over the course of the project. | A commercialization plan is expected in the application: a realistic plan (extending beyond the U44 Phase II) which outlines how and when full commercialization can be accomplished. |
Examples of Activities for BPN-Biologics Projects(pdf, 176 KB)
Example of Product Development Swim Lane BPN-Biologics(pdf, 194 KB)
FDA CBER Standard Operating Procedures and Policies (SOPPs)
Example Target Product Profiles:
Target Product Profile (TPP)(pdf, 189 KB)
Example Agent Profiles:
Example Antibody Profile(pdf, 100 KB)
Example Antisense Oligonucleotide Profile(pdf, 105 KB)
Example Viral Gene Therapy Profile(pdf, 118 KB)
Example Peptide or Protein Profile(pdf, 100 KB)
