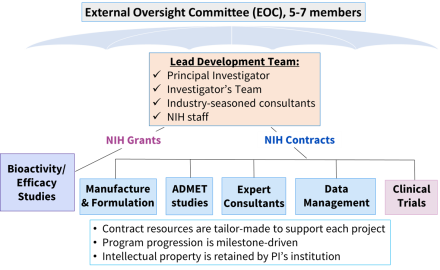
Successful applicants to BPN-Biologics receive non-dilutive funding for activities to be conducted in their own laboratories, in-kind access to contracted biotherapeutic development services, in-kind access to biotherapeutic development consultants, and retain intellectual property rights for biotherapeutic drug candidates developed through the BPN-Biologics program.
The Lead Development Team (LDT) is highly collaborative in nature and is the cornerstone of the BPN-Biologics infrastructure. The Principal Investigator and a Lead Consultant co-chair the LDT. The PI’s research team works closely with highly experienced consultants and BPN staff, holding regularly scheduled teleconferences to propose project milestones, design experiments, and review data. Decisions are made by consensus, within budgetary constraints.
Biotherapeutic development services are offered through NIH-funded Contract Research Organizations (CROs) specializing in ADME, toxicology, formulations development, GMP synthesis to enable IND-filing, and Phase I clinical testing. A UG3/UH3 or U44 grant award is provided to fund studies conducted by the Principal Investigator and associated personnel.